The compressibility factor Z for an ideal gas will be

The compressibility factor Z for an ideal gas will be

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
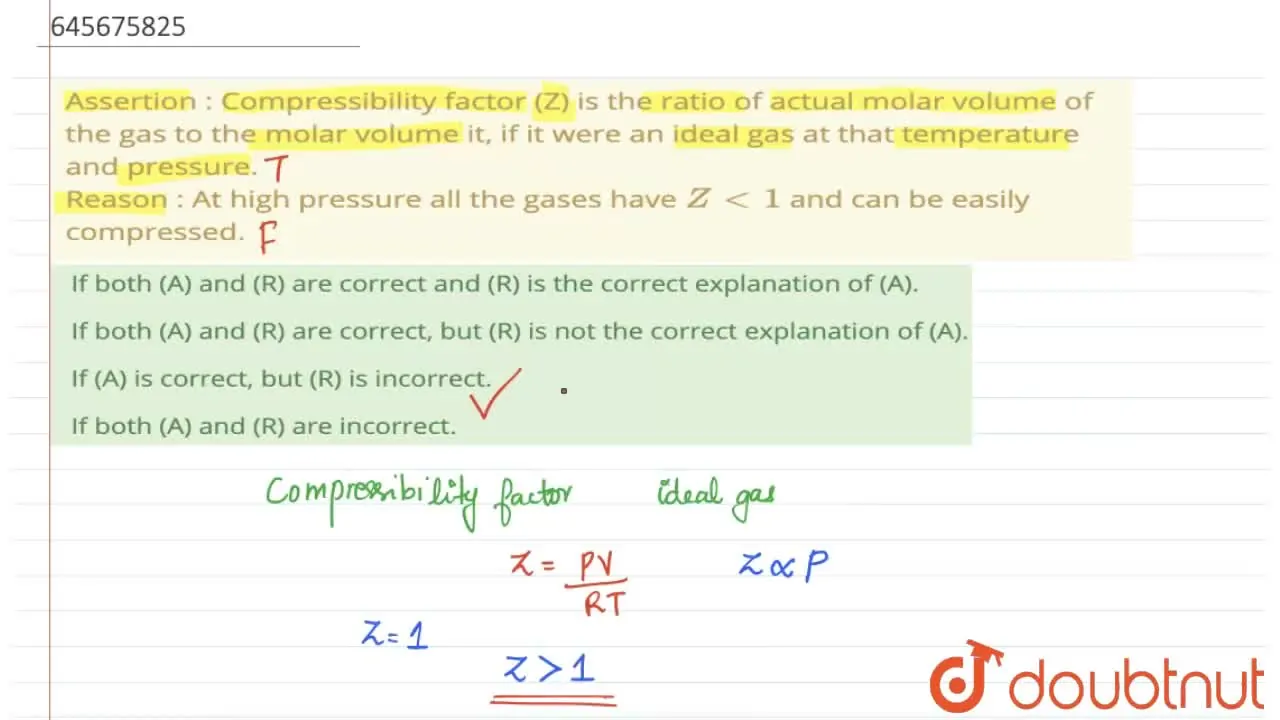
Malayalam] If (A) is correct, but (R) is incorrect.
Ideal gases and real gases are compressible or not compressible what is the compressible factor for real gases and ideal gases.

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Chemistry Desk: Effect of Pressure

MATHS This section contains FIVE questions. The answer to each

How many Balmer lines in the spectrum will be observed when electrons

68. The compressibility factor (z) an ideal gas is equal to which of the following values? (A) Zero (B) Less than one (C) Equal to one

Question ( 13 quad ) QnDirections: The answer to the following

Compressibility Factor Charts - Wolfram Demonstrations Project
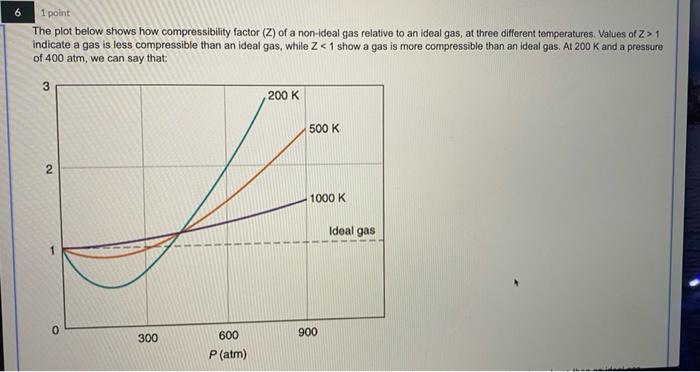
Solved 6 1 point The plot below shows how compressibility
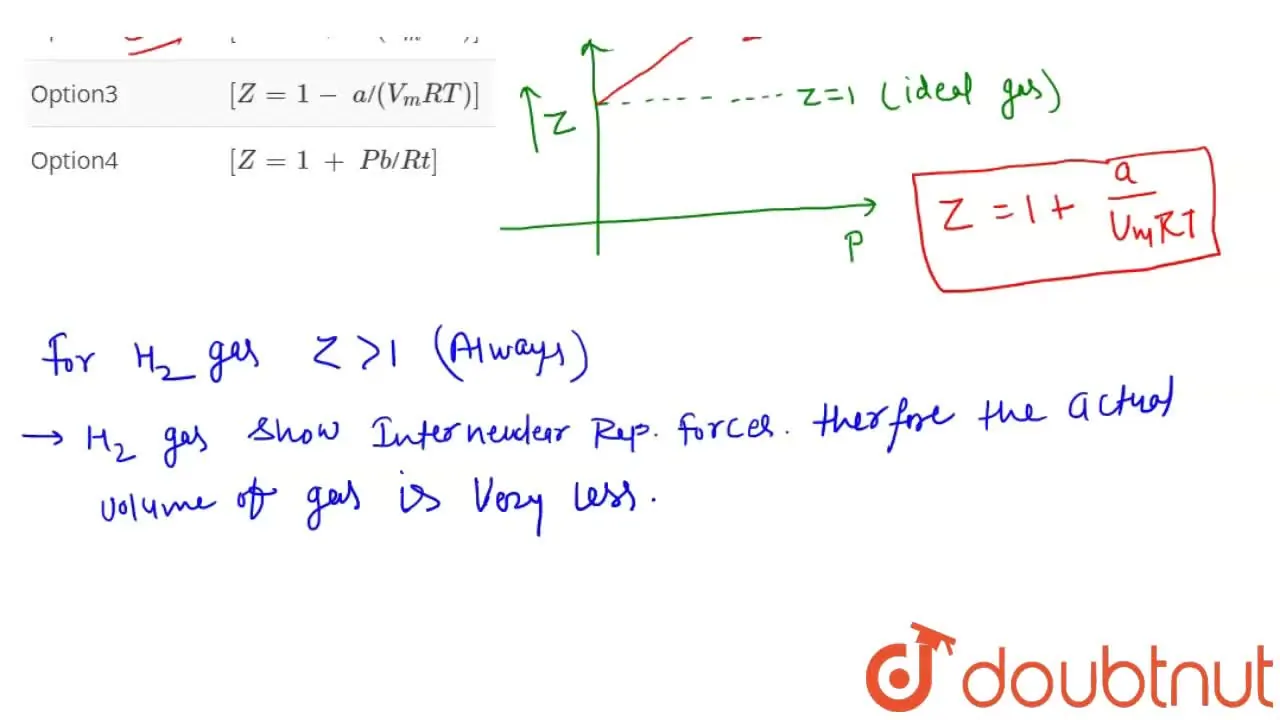
Compressibility factor (Z) for H2 (g) at STP is

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

For a non-ideal gas, the compressibility factor (Z) is defined as: Z










