Accelerated Aging: The Medical Package Testing Method that

Accelerated aging is a medical package testing method that can accurately assess packaging durability by simulating environmental factors such as heat and humidity.

Accelerated Aging Package Testing at Keystone Compliance

Packaging Accelerated Aging Studies

Accelerated Aging Shelf Life Testing
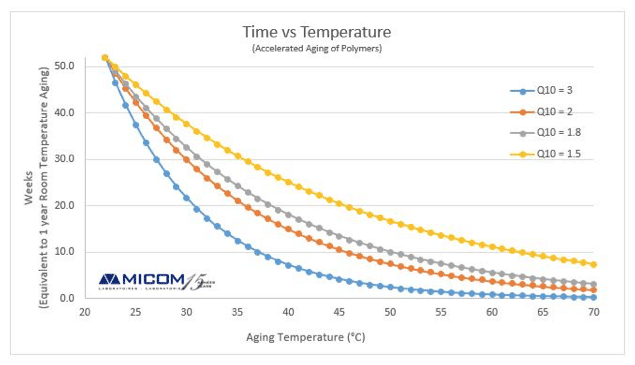
ASTM F1980 Is An Accelerated Aging Test Used For Medical Devices

Medical Device Packaging Validation

Accelerated Aging Testing [Medical Device Accelerated Aging]

Overview of Accelerated and Real Time Aging's Role in Package Validation
The Accelerated Aging Study simulates the effects of aging, weathering and handling by subjecting sterile packaged product to conditions considered

Accelerated Aging Study

Provided Ozone Concentration Environment Electric Wire Aging Test Equipment - China Aging Testing Machine, Ozone Aging Testing Equipment

ASTM F1980-99e1 - Standard Guide for Accelerated Aging of Sterile Medical Device Packages

Accelerated Aging Testing [Medical Device Accelerated Aging]






